Equal Opportunity Infection: Clostridium difficile
Traditionally described as a nosocomial infection affecting the elderly population and those who have recently been on antibiotics, CDI is growing in cost, prevalence, and severity.
Clostridium difficileis a prevalent, gram-positive anaerobe that is one of the most common causes of infectious diarrhea in the United States.1,2,3Transmitted primarily via the fecal-oral route,C. difficilecan overcome normal gastrointestinal (GI) microbes and generate infection with its toxin production.3-5C. difficileinfection (CDI) may manifest in a wide symptomatic range, from mild diarrhea to life-threatening toxic megacolon or even death.3,5Traditionally described as a nosocomial infection affecting the elderly population and those who have recently been on antibiotics, CDI is growing in cost, prevalence, and severity.1,2,4,6,7
Half a million Americans receive a diagnosis of CDI each year. Although many CDIs can be linked to hospitalization or long-term-care facilities and are defined as health care—associatedC. difficileinfections (HA-CDIs), an increasingly significant number of people are acquiring CDIs outside the hospital or an institution.1,2These infections are termed community-acquiredC. difficileinfection (CA-CDI) and can affect people who are younger, possess fewer comorbidities, have fewer health care exposures, and use antibiotics less frequently than their counterparts.2,7-9CA-CDIs are generally defined with the following criterion (in addition to a CDI diagnosis): symptom onset in the community before hospitalization or symptom onset within 48 hours of hospitalization when the patient has had no health care facility admissions within the previous 12 weeks.8-10Estimates show that CA-CDIs may account for 3% to greater than half of total CDIs.1,4,9On average, a single episode may cost more than $5,000.11
RISK FACTORS/POTENTIAL CAUSES
Risk factors for HA-CDIs are entrenched, consisting of increasing age (65 or older),12,13 proton pump inhibitor (PPI) or antibiotic use, GI surgery, immuno-suppression, lengthy health care stays, and complex comorbidities.1,14,15Patients who receive care within the home environment are more likely to be seen in the outpatient arena and therefore increase the probability for CA-CDI in this population.9

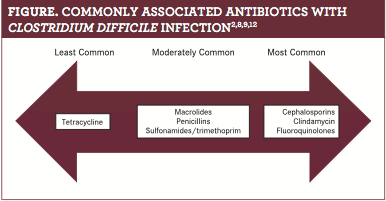
CA-CDIs represent a greater challenge in terms of these patients being generally younger and not possessing many of the common risk factors, com- pared with those with HA-CDI.8Despite the differences between the 2 types of CDI, common risk factors include increased prescriptions for antibiotics and subsequent usage. Patients with HA-CDIs have antibiotic use as high as 90% in the 12 weeks prior to diagnosis,16whereas patients with CA-CDI have lower antibiotic use, ranging from 25% to 60% in the same time frame, based on an analysis of available studies. Commonly prescribed antibiotics for CA-CDI include cephalosporins, clindamycin, and fluoroquinolones. Antibiotics less commonly associated with CA-CDI are macrolides, penicillins, and sulfonamides/trimethoprim. Tetracycline, however, was noted to produce no predisposition to CA-CDI and even protects against HA-CDI development (figure).2,8,9,12
The lack of an identifiable specific catalyst for the increased prevalence of CA-CDIs has prompted ascertaining other potential reasons for the emergence. The results of one study found that more than 80% of patients with CA-CDI had prior contact with an outpatient health care facility (ie, dental office, emergency department [ED], family practice office, specialty clinic, etc) as opposed to hospitalization.5Several positiveC. difficilecultures in the outpatient environment were discovered, which included about 10% of individual rooms in EDs, outpatient clinics, and specialty clinics. Total samples were positive in about 5% of all samples taken.6Suggestions also highlighted new unidentified sources, additional asymptomatic carriers in the community triggering more person-to-person transmission, the epidemicC. difficilestrain, more use of gastric acid—suppressing medications, and food and water contamination.9,10C. difficilecolonization in healthy adults who were not recently hospitalized is uncommon. However, colonization may occur in 25% to slightly more than 50% of hospitalized patients. Asymptomatic carriage was tied to recent use of antibiotics and prior CDI.9,15Asymptomatic carriers outnumber patients with CA-CDI by more than 3 times in the community. It is likely that 70% of children under 2 years may be colonized withC. difficile,and contact with these children is related to a greater risk of CA-CDI development.1,5,7,15Gastric acid suppression’s purpose in CA-CDI is debatable, as inconsistent evidence exists to determine whetherC. difficilespores are destroyed by stomach acid.Interestingly, PPIs were more likely to be used in patients with CA-CDI who had not had recent antibiotic use, by contrast to those patients with CA-CDI who had taken antibiotics recently. Increased providers’ recognition of potential community-based infectious diarrhea and implementation ofC. difficilestool tests may also be contributing to the higher CA-CDI prevalence.9
ETIOLOGY/TRANSMISSION
C. difficileis a pathogenic, spore-forming bacterium commonly found in the environment. Its spores are shed in the feces of colonized or infected individuals. Transmitted primarily through the fecal-oral route, the spores may spread from the hands of the infected person to the hands of caregivers. In addition, the spores may contaminate the environment and adhere to health care equipment, such as bedpans, beds, blood pressure cuffs, the floor, furniture, stetho- scopes, and toilet seats. Their life span can be many months, and they are challenging to eradicate. The spores are resistant to commonly used cleaning agents, which, in addition to heat, can expand the range of exposure. Spores become infectious when consumption and germination occur. Ingested spores become activated when normal GI flora is overcome, prompting the spores’ production of 2 toxins, A and B, which may cause colitis.1,3,9
CLINICAL FEATURES/DIAGNOSIS
Conventional CDI symptoms include abdominal pain or tenderness, fever, loss of appetite, nausea, and watery diarrhea.1 Patients with CA-CDI tend to have milder diseases, shorter hospitalizations, and lower mortality than those with HA-CDI.1,8,9,17However, the risk for adverse outcomes after CA-CDI still exists, and those comprise hospitalization, infection recurrence, and colectomy.2
CDI diagnosis is the same regardless of where the disease was acquired. Testing is recommended for CDI if a patient has 3 or more loose stools in a 24-to-48-hour period.5,10Testing consists of cell culture, neutralization tests, and cytotoxicity tests,12although many sources cite stool cultures as the gold standard. However, stool cultures may give false-positive results because of nontoxigenicC. difficilestrains.1,5,12An enzyme immunoassay (EIA) is an alternative test for CDI and can differentiate whether toxins A or B are present and for the glutamate dehydrogenase antigen. If the EIA results are inconclusive, polymerase chain reaction should be done to detectC. difficiletoxigenic genes. Repeat testing is not recommended unless the patient’s condition deteriorates, especially in asymptomatic individuals.1,5
TREATMENT/EMERGING THERAPIES
The severity of CA-CDI determines treatment choices, which may include, if possible, discontinuing the antibiotic causing symptoms. If that is not feasible, another option is to transfer the patient to an antibiotic appropriate for treatment but less likely to cause CDI, especially in those patients with further known risk factors.8Evidence shows that metronidazole and vancomycin are similarly efficacious in treating mild to moderate infections, but the latter is better for severe infections.5,11A newer antibiotic approved for CDI is fidaxomicin, which is equal to vancomycin in terms of treatment outcomes. Fidaxomicin is more effective in the reduction of recurrence rates. It is more expensive than metronidazole and vancomycin, but it should be acknowledged as a recourse for those patients with a greater chance of recurrence.1,9,11,18Other options are therapies, such as intravenous immunoglobulins and nitazoxanide. Antidiarrheal agents, such as loperamide, are not recommended, but additional research is necessary.1
Several nonantibiotic options are evolving as interventions. Probiotics have demonstrated possibilities in CA-CDI treatment. Probiotics may also be helpful in CDI prevention when taken for the duration of antibiotic therapy. The benefit appears to be greater for younger patients, as opposed to elderly patients taking antibiotics.5The evidence is limited, though some research on probiotics is in clinical trials.1Other nonantibiotic treatments in clinical trials include monoclonal antibodies and a vaccine, which has shown promise in rodents.1,19
RECURRENCE
Although CA-CDI is treatable, recurrences do occur, most often within 3 to 10 days after finishing antibiotic therapy. Recurrence may be the result of inadequate eradication ofC. difficilethrough antibiotic treatment or insufficient antibody production to theC. difficilebacterial toxins.1,18Nearly a quarter of patients develop a recurrence.1,3,11,18After the first episode of recurrence, the risk for a subsequent recurrence rises to 45%, and after 2 recurrences, the chances increase to 65%.11,18Recurrences are linked to increased health care costs, decreased quality of life, and higher morbidity.18
The same antibiotic used to treat the initial infection may be used to treat the first recurrence, depending on the CDI severity. For second or third recurrences, a vancomycin taper or a fidaxomicin is recommended.1,18Metronidazole should not be used in these instances because of the potential for neurotoxic adverse effects.18

If antibiotics fail, fecal microbiota transplantation (FMT), a highly successful treatment for recurrent CDI, may be used. FMT is considered first-line therapy for patients with more than 3 CDI recurrences. The procedure consists of transplanting fecal matter from a healthy donor into a patient’s GI tract through a colonoscopy, an enema, or a nasogastric tube. FMT is very effective in treating recurrences, as it contributes to the restoration of a balance of normal GI microbes.1,18If a patient has repeated recurrences or a continuing severe infection, consultation and referral to an infectious disease
specialist is indicated.1
PREVENTION
CA-CDI is challenging to prevent, but several methods to decrease transmission exist. Alcohol-based rubs and hand-sanitizing foams do not necessarily killC. difficilespores, but handwashing with soap and water is an established and documented effective infection control measure.1Both patients and providers need to be informed about CA-CDI risk linked with antibiotic prescriptions.8Providers need to systematically complete a comprehensive evaluation regarding a patient’s need for antibiotics, especially with the identified increase in antibiotic resistance.1
As CA-CDI emerges, infected individuals may require hospital admission. A risk that needs to be considered is that the individual’s home environment is contaminated before admission. The individual’s home must be thoroughly cleaned and disinfected with sporicidal agents, such as bleach, to prevent the patient’s returning to aC. difficile—contaminated environment and risking reinfection.5Environmental cleaning is frequently inadequate in inpatient settings and may be even worse in the outpatient arena because of high patient turnover.6
CONCLUSION
CA-CDI is emerging as a major health threat, especially as CDI has long been a nosocomial infection and patients with CA-CDI lack many of the traditional CDI risk factors. Antibiotic stewardship, handwashing, and patient/provider education are helpful tools to stem transmission of the disease. Careful attention is required for any patient presenting with suspected CA-CDI for appropriate education, testing, and treatment to curb its spread.
Jenna Herman, DNP, APRN, FNP-BC, is the family nurse practitioner program coordinator and an assistant professor at the University of
 Mary in Bismarck, North Dakota. Her clinical practice includes the emergency department of a level II trauma center, correctional medicine, and locum tenens in primary care clinics and hospitals across rural North Dakota.
References
1. Juneau C, Mendias EP, Wagal N, Loffelholz M, Savidge T, Croisant S, Dann SM. Community-acquiredClostridium difficileinfection: Awareness and clinical implications.The Journal for Nurse Practitioners.2013;9(1):1-6.
2. Kuntz JL, Chrischilles EA, Pendergast JF, Herwaldt LA, Polgreen PM. Incidence of and risk factors for community associatedClostridium difficileinfection: A nested case-control study.BMC Infectious Diseases.2011;11(194).
3. Nazarko L.Clostridium difficilein the community: Time to clean up.British Journal of Community Nursing.2014;19(10):512-513.
4. Hensgens MPM, Keessen EC, Squire MM, Riley TV, Koene MGJ, de Boer E, Lipman LJA, Kuijper EJ.Clostridium difï¬cileinfection in the community: A zoonotic disease?Clinical Microbiology and Infection. 2012;18(7):635-645.
5. Kim G, Zhu NA. Community-acquiredClostridium difficileinfection.Can Fam Physician.2017;63:131-2.
6. Jury LA, Sitzlar B, Kundrapu S, Cadnum JL, Summers KM, Muganda CP, Deshpande A, Sethi AK, Donskey CJ. Outpatient healthcare settings and transmission ofClostridium difficile. PLOS ONE.2013;8(7):e70175.
7. Tschudin-Sutter S, Tamma P, Naegel AN, Speck KA, Milstone AM, Perl TM. Distinguishing community-associated from hospital-associatedClostridium difficileinfections in children: Implications for public health surveillance.Clinical Infectious Diseases. 2013;57(12):1665—1672.https://doi.org/10.1093/cid/cit581
8. Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DDK, Sferra TJ, Hernadez AV, Donskey CJ. Community-associatedClostridium difficileinfection and antibiotics: A meta-analysis.Journal of Antimicrobial Chemotherapy.2013;68(1):1951-1961.https://doi.org/10.1093/jac/dkt129
9. Gupta A, Khann S. Community-acquiredClostridium difficileinfection: An increasing public health threat.Infection and Drug Resistance.2014;7:63—72.
10. Khanna S, Pardi DS, Aronson SL, Kammer PP, Baddour LM. Outcomes in community-acquiredClostridium difï¬cileinfection.Aliment Pharmacol Ther.2012;35:613—618.
11. Burton HE, Mitchell SA, Watt M. A systematic literature review of economic evaluation of antibiotic treatments forClostridium difficileinfection.PharmacoEconomics.2017;35:1123—1140.
12. Yoldaş O, Altındiş M, Cufali D, Aşık G, Keşli R. A diagnostic algorithm for the detection ofClostridium difficile-associated diarrhea.Balkan Med J. 2016;33:80-86.
13. Pechal A, Lin K, Allen S, Reveles K. National age group trends inClostridium difficileinfection incidence and health outcomes in United States community hospitals.BMC Infectious Diseases. 2016;16:682 DOI 10.1186/s12879-016-2027-8
14. Sahil K, Pardi DS, Aronson SL, Kammer PP, Oresnstein R, St Sauver JL, Harmsen WS, Zinsmeister AR. The epidemiology of community-acquiredClostridium difficileinfection: A population-based study.The American Journal of Gastroenterology.2012;107:89-95.
15. Nissle K, Kopf D, Rosler A. Asymptomatic and yet C. difficile-toxin positive? Prevalence and risk factors of carriers of toxigenicClostridium difficileamong geriatric in-patients.BMC Geriatrics.2016;16:185. DOI 10.1186/s12877-016-0358-3
16. Gerding DN, Young VB.Clostridium difficileinfection.Wound management principles. In: Bennet J, Dolin R, Blaser MJ, eds.Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Disease..8thed. New York, NY: Elsevier Saunders; 2015.
17. Reveles KR, Pugh MJV, Lawson KA, Mortensen EM, Koeller JM, Argamany JR, Frei CR. Shift to community-onsetClostridium difficileinfection in the national Veterans Health Administration, 2003-2014.American Journal of Infection Control.2017;1-6.
18. Baro E, Galperine T, Denies F, Lannoy D, Lenne X, Odou P, Guery B, Dervau B. Cost-effectiveness analysis of five competing strategies for the management of multiple recurrent community onsetClostridium difficileinfection in France.PLOS ONE.2017;12(1). doi:10.1371/journal. pone.0170258
19. Zhang Z, Cai J, Yu B, Hua Y, Lau CC, Yi-Tsun R, Kao T, Sze KH, Yuen KY, Huang JD. A DNA vaccine targeting TcdA and TcdB induces protective immunity againstClostridium difficile. BMC Infectious Diseases.2016;16:596.
Knock Out Aches and Pains From Cold
October 30th 2019The symptoms associated with colds, most commonly congestion, coughing, sneezing, and sore throats, are the body's response when a virus exerts its effects on the immune system. Cold symptoms peak at about 1 to 2 days and last 7 to 10 days but can last up to 3 weeks.
COPD: Should a Clinician Treat or Refer?
October 27th 2019The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines the condition as follows: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.â€
Diabetic Ketoacidosis Is Preventable With Proper Treatment
October 24th 2019Cancer, diabetes, and heart disease account for a large portion of the $3.3 trillion annual US health care expenditures. In fact, 90% of these expenditures are due to chronic conditions. About 23 million people in the United States have diabetes, 7 million have undiagnosed diabetes, and 83 million have prediabetes.
What Are the Latest Influenza Vaccine Recommendations?
October 21st 2019Clinicians should recommend routine yearly influenza vaccinations for everyone 6 months or older who has no contraindications for the 2019-2020 influenza season starting at the end of October, according to the Advisory Committee on Immunization Practices.
What Is the Best Way to Treat Pharyngitis?
October 18th 2019There are many different causes of throat discomfort, but patients commonly associate a sore throat with an infection and may think that they need antibiotics. This unfortunately leads to unnecessary antibiotic prescribing when clinicians do not apply evidence-based practice.

